Previous Cases
Xeljanz
Xeljanz (tofacitinib) is manufactured by Pfizer and was approved by the FDA for the treatment of moderate to severe rheumatoid arthritis (RA), particularly in patients that do not respond well to other forms of treatment. The drug works by blocking the enzymes responsible for the joint inflammation that RA patients suffer.
Nuvasive
The NuVasive Precice system is an implantable, adjustable magnetic device that treats people with limb length discrepancy, a condition where the length of the tibia or femur are unequal. In February 2021, NuVasive removed stainless steel Precice implants from the market and recalled titanium Precice implants.

IVC Filters
An IVC filter is a kind of small strainer used to filter out blood clots or keep them from forming and becoming pulmonary embolisms (i.e., blood clots traveling to the lungs). The IVF or inferior vena cava is a large vein entering the right atrium (upper chamber) of the heart. It delivers oxygen-depleted blood from the legs, the pelvis, and the abdomen back to the heart.

JUUL & E-Cigarettes
JUUL and e-cigarette use, especially among children and teens, has become an epidemic both in our community and nationwide. The e-cigarette JUUL is said to be a smoking “cessation” device for adults, but its growing popularity among children and teenagers, and the company’s marketing tactics and fruity flavors targeted to younger users.

Hurricane Michael Insurance Claims
Hurricane Michael came ashore in October 2018 as the strongest hurricane to hit the Florida Panhandle in recorded history. It pummeled much of the southeastern United States, leaving millions without power and causing damage estimated in the billions of dollars.

Human Trafficking
Human trafficking is one of the world’s fastest growing criminal industries. Traffickers use force, fraud or coercion to trap millions of people across the globe into lives of forced servitude. The majority of trafficking victims are women and girls, many of whom are targeted for sexual exploitation, the most common type of human trafficking.

Sexual Abuse in Schools
Colleges and universities may fail to adequately prevent and respond to sexual abuse and harassment. Recent media reports have revealed how institutions of higher education have engaged in cover-ups of chronic sexual abuse perpetrated by some of their most respected employees.

Sexual Abuse in the Boy Scouts
The Boy Scouts of America has long maintained a list of leaders and volunteers it deems ineligible to work with the organization. Many of those ineligible have been listed due to allegations of sexual abuse against children.

Sexual Abuse in Sports
Amateur sports organizations offer young athletes opportunities to excel in physical endeavors. Unfortunately, they also offer sexual predators access to victims. Perhaps the most widely known sexual predator in sports is Larry Nassar. The former physician for the USA Gymnastics Team is accused of sexually abusing over three hundred women and girls during his tenure.

Proton Pump Inhibitors
For nearly a decade, manufacturers of PPI’s have been on notice that these drugs can cause kidney disease and injury, including acute interstitial nephritis. Finally, in December of 2014, manufacturers changed their package inserts and labels to warn of an association between PPI’s and interstitial nephritis, diagnosed by kidney biopsy.
Zantac & Ranitidine
Zantac and its generic, ranitidine, have been found to contain the cancer-causing chemical NDMA (N-nitrosodimethylamine). This chemical is used in gasoline, rocket fuel, as a stabilizer in industrial materials, and as an additive to lubricants. Zantac is sold by the manufacturer Sanofi, as well as generically under the name ranitidine.
Belviq Lawsuit
Belviq was approved by the FDA in 2012 for use as an add-on therapy to help aid weight loss, along with diet and exercise, in people who were obese or overweight. Belviq increases feelings of fullness so that people eat less. It’s available as a tablet (Belviq) and an extended-release tablet (Belviq XR).
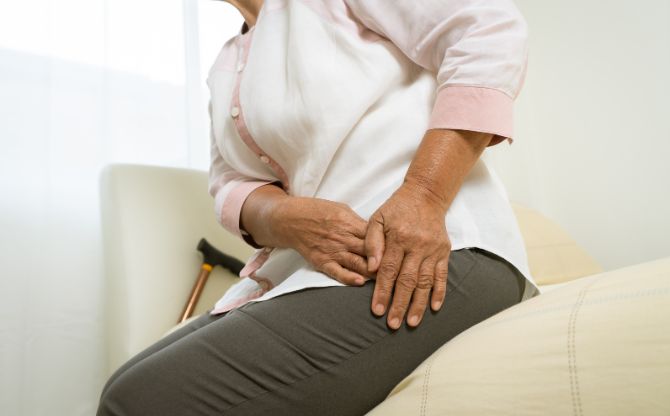
Hip Implants
Multiple manufacturers of hip implant devices and hip replacement systems have had to issue recalls of their products due to high rates of failure. In some instances, the defective implants were prone to breakage and failure, while other hip systems were found to cause a dangerous condition known as metallosis.

Whole Life Insurance
Whole Life Insurance policy holders were overcharged for varying amounts of time. Whole life insurance policies generally cover the policyholder’s entire lifespan. The rates for this coverage, typically much higher than the rates for term insurance, are determined by a number of set factors, such as the insured’s aged and sex.

Stryker Tritanium Hip Implants
Due to it’s highly porous surface, the Tritanium components were designed to increase integration with natural bone and thereby increase durability of the hip implant without the use of cement as is used in traditional hip replacement devices. Unfortunately, early case studies are reporting poor outcomes of the Tritanium devices, including early loosening of the Tritanium cup.

Essure
Essure is a permanently implanted birth control device. Essure is unique in that it is the only permanent birth control device that does not require a surgical incision. It was approved for use by the FDA in 2002. The device is deposited into the fallopian tubes via flexible coils inserted into the vagina and cervix.

Knee Replacement
More than half a million knee replacements are performed every year in the U.S. Nearly all knee implant systems, including related component parts, however, are not approved by the FDA. This is because manufacturers do not want to incur the cost and delays associated with securing “FDA approval” which requires that the company test to determine device safety and efficacy.

